


OMEGA Pharmacy colleges offers- Master of Pharmacy (M.Pharm) program with a specialization in Pharmaceutical Chemistry is a postgraduate degree in pharmacy that focuses on the study of the chemical aspects of pharmaceuticals and drug development. This program is typically regulated by the Pharmacy Council of India (PCI) and affiliated to Osmania University.
Below are the course details for an M.Pharm in Pharmaceutical Chemistry according to PCI guidelines.
Duration: The M.Pharm in Pharmaceutical Chemistry program usually has duration of 2 years, divided into four semesters.
Eligibility Criteria: To be eligible for admission to this program, candidates typically need to have completed a Bachelor of Pharmacy (B.Pharm) degree from a PCI-approved institution with a minimum aggregate score, often around 55% to 60%. Some institutions may also require candidates to have a valid Graduate Pharmacy Aptitude Test (GPAT) score OR POST GRADUATION COMMON ENTRENCE TEST (PGCET).
Course Curriculum: The curriculum for M.Pharm in Pharmaceutical Chemistry may include the following subjects and topics:
Pharmaceutical Organic Chemistry: In-depth study of organic chemistry principles as they apply to drug synthesis and design.
Medicinal Chemistry: Study of the design, synthesis, and development of pharmaceutical agents with an emphasis on drug-receptor interactions.
Pharmaceutical Analysis: Techniques and methods for the qualitative and quantitative analysis of pharmaceutical substances.
Pharmaceutical Biochemistry: Understanding the biochemical aspects of drug action and metabolism in the body.
Pharmacology: An introduction to the study of drugs, their mechanisms of action, and their effects on the body.
Pharmacokinetics and Pharmacodynamics: Study of drug absorption, distribution, metabolism, and excretion (ADME) as well as drug-receptor interactions.
Pharmaceutical Formulation and Technology: The development and formulation of various dosage forms like tablets, capsules, and injections.
Instrumental Analysis: Utilization of various analytical instruments in pharmaceutical analysis.
Research Methodology and Project Work: Training in research methodologies and a research project in pharmaceutical chemistry.
Regulatory Affairs and Quality Assurance: Understanding of pharmaceutical regulations and quality control measures.
Computer-Aided Drug Design: Use of computational tools and software for drug discovery and design.
Industrial Training: Exposure to the pharmaceutical industry through internships and practical training.
Assessment: Evaluation in M.Pharm programs is typically based on a combination of written exams, practical examinations, project work, and sometimes viva voce (oral examinations).
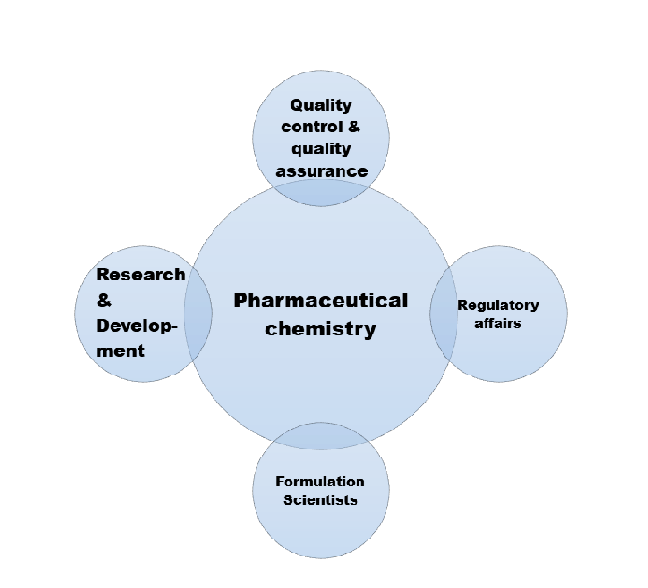
Career Opportunities: After completing M.Pharm in Pharmaceutical Chemistry, graduates can pursue careers in various sectors, including pharmaceutical research and development, quality control and assurance, academia, regulatory affairs, and more. They can work as research scientists, analytical chemists, formulation scientists, and in various roles in the pharmaceutical industry.